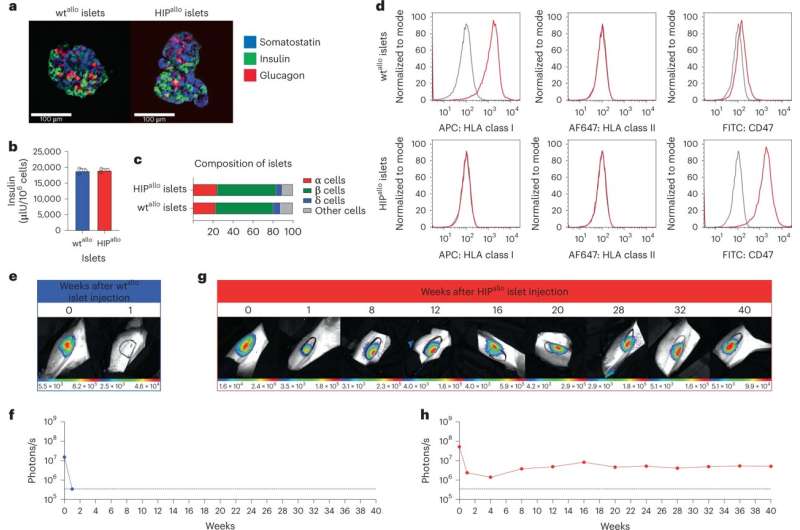
Allogeneic HIP islets achieve prolonged survival. a, Immunofluorescence images of dissociated and aggregated wtthere and HIPthere Islets from rhesus monkeys (representative images). b, In vitro insulin secretion of wtthere and HIPthere Islets (mean ± sd, triplicates). c, composition of wtthere and HIPthere Islets of α, β, δ and other cells (mean, triplicates). d, Flow cytometry histograms for HLA class I and II and rhesus CD47 in wt.there and HIPthere Islands (representative images). eh, survival of the FLuc+ wtthere and HIPthere Islets in allogeneic rhesus monkeys were followed by BLI (one animal each). Credit: Nature Biotechnology (2023) DOI: 10.1038/s41587-023-01784-x
Sana Biotechnology, Inc. of California. Researchers have made significant progress in realizing the promise of stem cell therapy: stem cells from an immunologically incompatible donor that do not trigger an immune response.
Published in the journal “Hypoimmune induced pluripotent stem cells are long-lived in fully immunocompetent, allogeneic rhesus macaques” Nature BiotechnologyResearchers describe how they cloned a line of hypoimmune pluripotent (HIP) stem cells to bypass normal rejection and destruction barriers to therapeutic use.
In an experimental setting, hypoimmune pluripotent cells did not trigger an immune cell response. They were resistant to cytotoxicity induced by wild-type stem cells transplanted alongside them, successfully evading direct detection and effects from enrichment of off-target threats.
HIP cells survived for up to 16 weeks (the entire test period) in fully immunocompetent allogeneic recipients and differentiated into several lineages, whereas wild-type cells were acutely rejected.
In a humanized diabetic mouse model, pancreatic differentiated human HIP cells showed evidence of improvement over four weeks. Mice were not immunosuppressed and were not matched for the cell types used.
Additional long-term testing of HIP cells found cell islets after 40 weeks in rhesus macaque recipients without immunosuppression, compared to an unedited wild-type version that was destroyed within a week.
Stem cells have the potential to revolutionize medicine because they can be manipulated to differentiate into different cell types, making them a promising source of new cells for transplantation or regenerative medicine. By introducing stem cells into damaged tissue or organs, it may be possible to regenerate healthy tissue and restore proper function. This has implications for developing new treatments for a variety of diseases, including cancer, heart disease and neurological disorders.
Human induced pluripotent stem cells (hIPSCs) were first created in 2007 by Kazutoshi Takahashi of Kyoto University in Japan and colleagues. Since then, researchers have been working to find ways to unlock the potential of these stem cells for therapeutic applications, but some obstacles have slowed their widespread clinical application.
Stem cells can proliferate like any other type of cell, creating new disease-free tissue. However, these autologous cells still have the chance to proliferate after transplantation, proliferate into the wrong tissue, or carry genetic mutations with off-target interactions, all of which can potentially lead to tumors. This problem may have direct knowledge-based solutions in cell line selection and maintenance, cell signaling, and other genomic aspects as stem cells move from research environments to more focused clinical applications.
Another problem is that human leukocyte antigens (HLAs), markers found on most cells used by the immune system to distinguish native cells from invading cells, allow invading cells to be targeted for destruction. Humans have thousands of variations of HLAs, which would then require engineering stem cells to match each individual, a process that is feasible but not scalable for mass therapeutics. Without a close match, the immune system will eliminate the stem cells introduced into the host.
Stem cells for the masses
Banks containing tens of thousands of HLA stem variants and millions of cells are a highly impractical way of solving the problem. If this is the only option, we can do it with current technology and it will eventually become available through a long, expensive journey down a paved road with good intentions.
A more viable solution that can still be banked is the HLA cloaking method. With just a few functional stem cell lines edited in a way that is hidden from plain view of the immune system, a pool of universal donor stem cells can be developed. Additionally, this approach will make it much easier to study, accelerate our understanding of some very versatile lines, and allow us to streamline regulatory approval for clinical trials and treatments.
The successful approach used by Sana Biotechnology is missing from the scientific research community. Allowing for unmatched HLAs in non-immunosuppressed patients overcomes many barriers between the potential future of stem cell therapies and the piles of research papers describing the routine application of revolutionary medicine.
More information:
Xiaomeng Hu et al, Hypoimmune-induced pluripotent stem cells are long-lived in fully immunocompetent, allogeneic rhesus macaques, Nature Biotechnology (2023) DOI: 10.1038/s41587-023-01784-x
© 2023 Science X Network
reference: Breakthrough research could bring stem cell therapy to the masses (2023, May 10) Retrieved 10 May 2023 from https://phys.org/news/2023-05-breakthrough-stem-cell-therapy-masses.html
This document is subject to copyright. No part may be reproduced without written permission, except in any fair dealing for the purpose of private study or research. Content is provided for informational purposes only.
#Breakthrough #research #bring #stem #cell #therapy #masses
